Add distilled water until volume is L. When the concentrations of the acid and its conjugate base that is the borate ions is exactly 11 the pH will be most difficult to change since any addition of hydrogen ions will only bond with the borate to form the undissociated boric acid.
Pa ACS reagent reag.

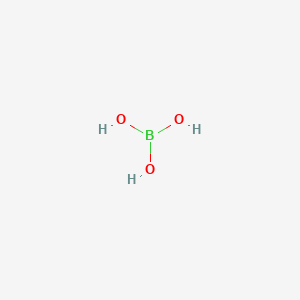
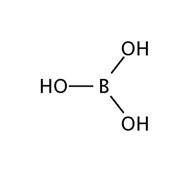
Boric acid buffer. Dilute to desired molarity with ddH 2 0. Boric acid is a weak acid. For example the bicarbonate buffering.
At 25C its pKa the pH at which buffering is strongest because the free H2. Find Sigma-Aldrich-31146 MSDS related peer-reviewed papers technical documents. The boric acid strong liquor high borate concentration is nearly saturated with sodium sulfate.
Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. The buffer solutions recommended by pharmacopoeia are called as official buffers. It contains boric acid potassium chloride and sodium hydroxide.
Boric acid 02M 1237 gm1 H 3 BO 3 MW 6183 Add boric acid to borax solution until desired pH is reached. Add 10 g of NaOH to the solution. In nature there are many systems that use buffering for pH regulation.
Eur buffer substance 998. Add dH2O until volume is 1 L. Lubrication when used as a colloidal suspension of nanoparticles dissolved in petroleum oil boric acid can be a very efficient lubricant for ceramic and metal surfaces.
The prepared solutions should be stored in chemically resistant glass. Solution B 005 m sodium tetraborate 10H 2 O 75 mL. A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa.
Complete solubility of sodium sulfate is maintained throughout the process by careful control of pH and temperature. The above link gives you the procedures to making the buffer. Although boric acid is known to complex with various polyalcohols it is often used as a component of buffer mixtures.
It contains potassium hydrogen phthalate and hydro chloric acid. Some eye drops contain borax or boric acid which are sources of boron these ingredients are included as buffers to make sure the medicine is not too acidic or alkaline and is comfortable when. It is a complex system bust since you want pH 9 that should be straightforwardUse the 100 mM concentration to.
To perform agarose gel electrophoresis recovery experiments of DNA fragments it is recommended to use TAE buffer. Volume per 500 mL of solution vv Solution A 02 m boric acid 175 mL. Electrochemical and spectrophotometric experiments have.
Perhaps the test writers wanted an interesting weak acid and conjugate base for the problem and either did not worry about the true complexity of the boric acid chemistry or were not aware of it. Borate Buffer pH 80 1 Reagent. This online resource may be cited as follows.
Adjust solution to final desired pH using HCl or NaOH. DNA-borate complexes are not observed in agarose gels because of the competition of the agarose gel fibers for the borate residues. The resulting agarose-borate complexes increase the negative charge.
Prepare 800 mL of dH2O in a suitable container. Now here are the twists. Add g of Boric Acid to the solution.
The temperature is dropped to 70 C in the first. Sodium Borate Buffer 1 M pH 85 Preparation AAT Bioquest Inc 27 Sep. Swimming-pool context the boric acid buffer system and the borate buffer system are exactly the same thing.
The boric acid component in the TBE buffer will affect the efficiency of DNA recovery and subsequent enzymatic reactions. Citrate Buffer sodium citrate-citric acid buffer pH 3-62. This buffer is five times the concentration used in the text.
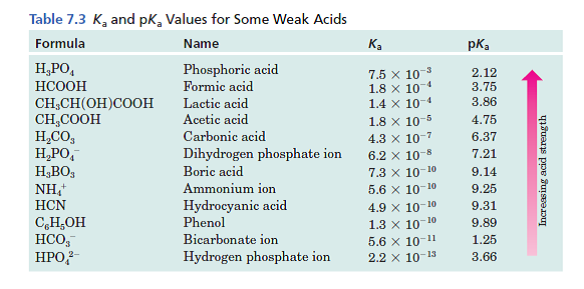
The reason why the borax solution is able to keep the system at around pH of 9 because the boric acid that is produced from this reaction has a pKa of 946. Add 6183 g of Boric Acid H3BO3 to the solution. Carbon dioxide-free water should be used for preparing buffer solutions and wherever water is mentioned for preparation of such solutions the use of carbon dioxide-free water is implied.
01 M borate buffer pH 74 MATERIALS Disodium tetraborate Na 2B 4O 710H 2O 954 g in 250 ml distilled water Boric acid 2473 g in 4 l distilled water METHOD Add approximately 115 ml borate solution to 4 l boric acid solution until pH reaches 74. Find boric acid buffer and related products for scientific research at MilliporeSigma. All the crystalline reagents except boric acid should be dried at 110 to 120C for 1 hour before use.
The strong liquor is filtered at 98 C and boric acid crystallized in two stages using continuous evaporative crystallizers. The boric acid component in TBE buffer will affect the efficiency of DNA recovery and subsequent enzymatic reactions. Dilute just before use.
Alkaline borate buffer. The borate ions in Tris-borate-EDTA TBE buffers interact with DNA to form highly charged DNA-borate complexes which are stable both in free solution and in polyacrylamide gels. PH buffer together with a conjugate base the acids borate is commonly used as a buffer system for swimming pools in the concentration range of 50 to 100 ppm boron equivalents.
The borate-boric acid buffer system may be the best approach. For agarose gel electrophoresis recovery experiments of DNA fragments it is recommended to use TAE buffer. Product name Buffer solution pH 1000 20C Boric acidPotassium chlorideSodium hydroxide 005 pH-units AVS TITRINORM Synonyms Not Available Other means of identification Not Available Relevant identified uses of the substance or mixture and uses advised against Relevant identified uses Use according to manufacturers directions.
Around pH 7 boric acid is the predominant form in aqueous solution. Its pH changes very little when a small amount of strong acid or base is added to it. In addition the authors of the questions may not be the ones who provided the solutions to the problems.

Cyclic Voltammograms In Deaerated Boric Acid Buffer Ph 6 5 And Borate Download Scientific Diagram

Buffer Solution Boric Acid Potassium Chloride Sodium Hydroxide Solution Traceable To Srm From Nist And Ptb

The Effect Of A Different Buffers Phosphate Buffer Borate Buffer Download Scientific Diagram

Pdf Boric Acid As A Swimming Pool Buffer Semantic Scholar

Preparation Of Phosphate And Alkaline Borate Buffer Solutions And Download Scientific Diagram

Proposed Mechanism For The Adsorption Of Boric Acid On Iron Oxide Download Scientific Diagram
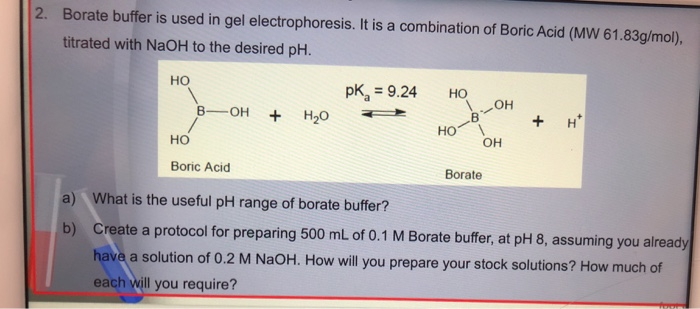
Solved 2 Borate Buffer Is Used In Gel Electrophoresis It Chegg Com
Boric Acid H3bo3 10043 35 3 P A Buffer Acs Iso Ph Eur Pack 25 Kg
File Tbe Buffer Svg Wikimedia Commons

Boric Acid 99 999 Trace Metals Basis 10043 35 3
Solved What Is The Ph Of A Boric Acid Buffer Solution Containing Chegg Com

Comparison Of Different Amounts Of Potassium Borate Buffer On The Download Scientific Diagram

How To Calculate The Composition Of A Borate Buffer With A Defined Ph Using The Henderson Hasselbalch Equation Chemistry Stack Exchange

File Tbe Buffer Svg Wikimedia Commons

Preparation Of Phosphate And Alkaline Borate Buffer Solutions And Download Scientific Diagram